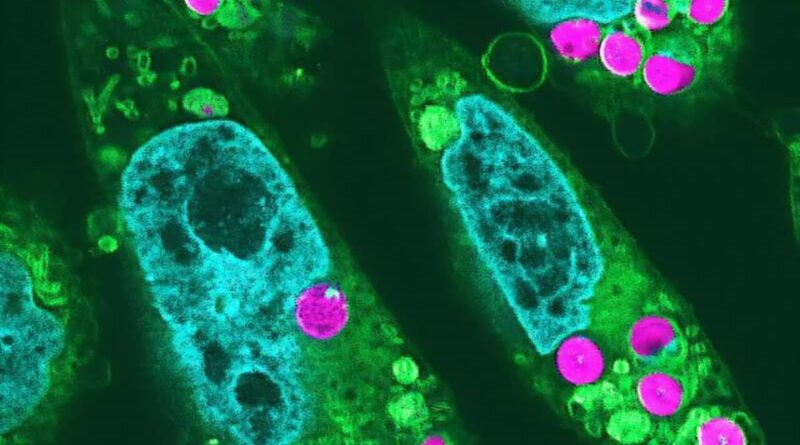
Advances when scientists made animal cells photosynthesize
In a world first that challenges what we thought we knew about biology, scientists have succeeded in making animal cells capable of photosynthesizing. The breakthrough promises to revolutionize medical research and increase production of lab-grown meat.
Photosynthesis is a biochemical process that plants, algae and certain bacteria use to convert light energy from the sun into food. This process—which occurs in specialized cells called chloroplasts—uses water and carbon dioxide and produces oxygen, as well as sugar that can be used by the plant.
“All living things on Earth, including humans, are able to survive because of photosynthesis,” Sachihiro Matsunaga, Professor of the Laboratory of Integrated Biology at the University of Tokyo who led the research, said. Newsweek. “Animal cells consume oxygen, consume and break down sugar, and release carbon dioxide. This reaction is completely different from photosynthesis.”
Scientists have been trying to make photosynthesis happen in animals since the 1970s.
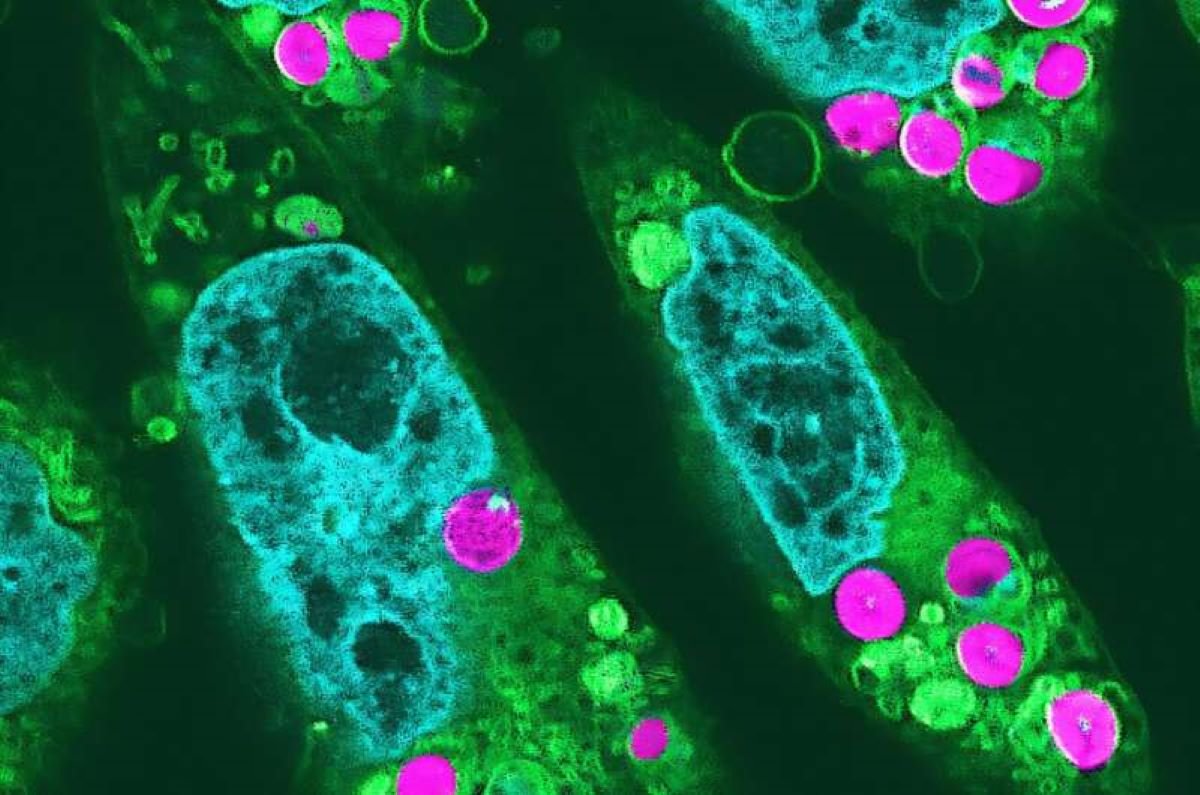
R. Aoki, Y. Inui, Y. Okabe et al. 2024/ Proceedings of the Japanese Academy, Series B
“If we can get even a fraction of photosynthesis to take place in animal cells, we can reduce oxygen consumption, reduce the amount of sugar consumed, and reduce carbon dioxide emissions,” Matsunaga said.
However, getting these chloroplasts into animal cells is too easy to say done.
“Animal cells recognize chloroplasts as foreign and destroy them immediately, so the chloroplasts did not work even when they were inserted into animal cells,” said Matsunaga. “As a result, after about 10 years, everyone stopped doing research. It became ‘common knowledge’ in the field of biology that the chloroplasts themselves they don’t work in animal cells.”
Besides looking like “cells”, chloroplasts themselves struggle to function in the environment of an animal cell.
“Almost all algae and plant species photosynthesize below 30 degrees Celsius,” Matsunaga said. “Most animal cells are grown at 37 degrees Celsius.”
Therefore, researchers had to look for chloroplasts that will be able to work in the warm environment of the animal cell.
“The success of this research is due to the isolation of chloroplasts from the schyzon, which grows in a hot volcanic spring in Italy at 42 degrees Celsius and has chloroplast activity even at 37 degrees Celsius ,” said Matsunaga.
“Animals”
Some animals have been able to use the energy of photosynthesis for themselves. The “leaf sheep” sea slug, for example, can absorb chloroplasts from the green algae it feeds on. This not only allows the leaves to photosynthesize, but also turns them bright green, which gives the appearance of an underwater artichoke.

Kittisak Songprakob/Getty
The next step was to prevent animal cells from rejecting chloroplasts as “foreign materials”.
“When chloroplasts are forcibly inserted into animal cells, they are recognized as foreign substances such as bacteria or viruses and are quickly destroyed,” Matsunaga said. “[However, our] chloroplasts were fed to animal cells as food, rather than forced into animal cells.
“Edible chloroplasts could be kept in an animal cell for at least two days, during which the first reaction of photosynthesis could be observed. This is the first time in the world that we have been able to detect the first reaction of photosynthesis in animal cells We have managed to overturn conventional biology.
Not only that, the animal cells that had chloroplasts showed an increased growth rate, suggesting that the chloroplasts provided an alternative source of food for the people who receive them.
“I was surprised because we were able to do something that no one had been able to do for 50 years and that all the biological researchers had already dedicated themselves,” said Matsunaga.
So, does this mean we will all be able to start photosynthesizing? No, we’re not there yet.
“Since our technology is adapted to the cells of advanced animals, the short way is to consider it useful for the development of [mini model organs for medical research] and synthetic meat,” Matsunaga said. “When the cells become multilayered, the inside of the cells [don’t get enough oxygen,] cell division stops, and an increase in size is not possible. However, if light is transferred into the cell mass, oxygen can be supplied by the captured chloroplasts, [low oxygen] the situation in the cell mass can be improved, and cell division can start again.”
In the future, this process could also show promise in medicine, for example if patients need more oxygen delivered to certain organs.
“If the goal of treatment is to distribute oxygen near the heart’s blood vessels in order to improve heart disease, it may be sufficient to install an LED light source near the heart,” said Matsunaga.
However, before that can happen, the team will need to keep the chloroplasts active for longer than just two days, as was the case in this experiment.
“In the future, we will improve our method so that chloroplasts can carry out photosynthesis in animal cells for as long as possible,” said Matsunaga.
Do you have any advice on science fiction that Newsweek should cover? Have a question about cellular engineering? Let us know at science@newsweek.com.
References
Aoki , R. , Inui , Y. , Okabe , Y. , Sato , M. , Takeda-Kamiya , N. , Toyooka , K. , Sawada , K. , Morita , H. , Genot , B. , Maruyama , S. . , et al., Tomo, T., Sonoike, K., & Matsunaga, S. (2024). Introduction of photosynthetically active algal chloroplasts into mammalian cells evolved to lead to photosynthesis in animals. Proceedings of the Japanese B-School Series.
https://doi.org/10.2183/pjab.100.035
#Advances #scientists #animal #cells #photosynthesize